Publications
Publications and Patents
Publications at NKU
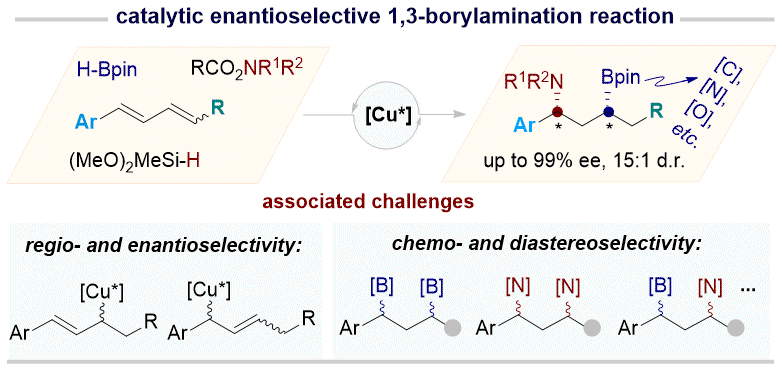
52. Enantioselective Construction of 1,3-Stereogenic Centers via 1,3-Borylamiantion of Conjugated Dienes
Zhu, Yu-Shen; Xu, Youzhi; Zhu, Ying-Ying; Huang Genping, Su, Bo* J. Am. Chem. Soc., 2026,148, 114-123.
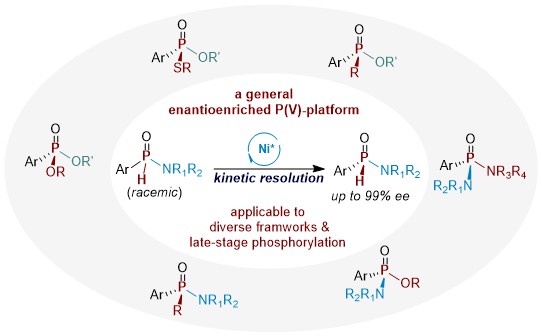
51. Enantioselective Synthesis of H-Phosphinamidates (selected as Hot Paper)
Ding, K.; Xue, J.J., Zou J.X.; Zhang, Q.W.*, and Su, B.* Angew. Chem. Int. Ed. 2026, 65, e22534.
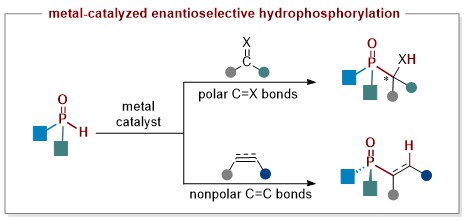
50. Copper-Catalyzed Enantioselective Hydrophosphorylation of Unactivated Alkynes (selected as Hot Paper; "Most Accessed Papers" in Oct. 2024)
Kang, J.;ǂ Ding, K.;ǂ Ren, S.-M.; Yang, W.-J.; Su, B.* Angew. Chem. Int. Ed. 2025, 64, e202415314. (ǂEqual contribution)
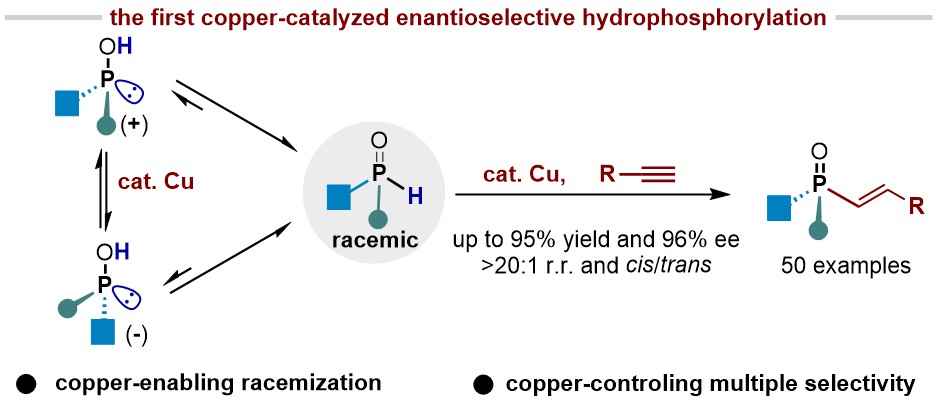
The enantioselective addition of H-P(O)R1R2 to alkynes (hydrophosphorylation) offers a direct and efficient pathway to P-chiral compounds; however, the enantioselective hydrophosphorylation of unactivated alkynes remains a significant challenge, typically relying on noble palladium catalysts and proceeding via a kinetic resolution mechanism with less than 50% conversion. To address these limitations, we recently achieved earth-abundant copper-catalyzed dynamic kinetic asymmetric hydrophosphorylation of alkynes, using a newly designed chiral 1,2-diamine ligand. This reaction is compatible with both aromatic and aliphatic terminal alkynes, yielding products with high yields (up to 95%), exclusive cis selectivity, and exceptional regio- and enantioselectivity (over 20:1 r.r. and up to 96% ee).
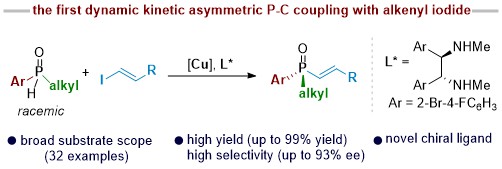
49. Dynamic Kinetic Asymmetric P-C Coupling of Secondary Phosphine Oxides with Alkenyl Iodides
Ren, S.-M.;ǂ Yang, W.-J.; ǂ Ding, K.; Kang, J.; Zou, J.-X.; and Su, B.* Org.Lett. 2025, 27, 6082-6087. (ǂEqual contribution)
48. Enantioselective 1,4-Borylamination Enabled by Copper Catalysis (Invited Manuscript)
Zhu, Yu-Shen; Su, Bo* Synlett, 2025, 36, 1441-1446.
47. Metal-Catalyzed Enantioselective Hydrophosphorylation Reactions (Invited Review)
Ding, K.; Zou J.X.; and Su, B.* Asian J. Org. Chem. 2025, 14, e00597. (ǂEqual contribution)
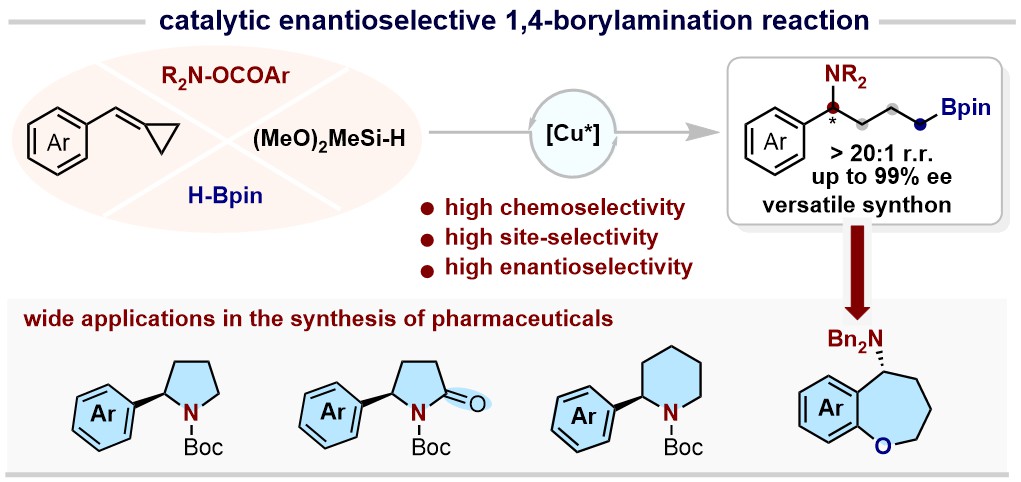
46. Enantioselective 1,4-Borylamination via Copper-Catalyzed Cascade Hydroborylation and Hydroamination of Arylidenecyclopropanes (Highlighted by Synfacts, Trends in Chem)
Zhu, Yu-Shen; Guo, Ya-Lin; Zhu, Ying-Ying; Su, Bo* J. Am. Chem. Soc., 2024, 146, 47, 32283–32291.
45. Enantioselective Synthesis of Secondary Homoallyl Borons by Copper-Catalyzed 1,1-Borylallylation of Terminal Alkynes
Liu, S.;ǂ Ding, K.;ǂ Su, B.* ACS Catal., 2024, 14, 12102-12109. (ǂEqual contribution)
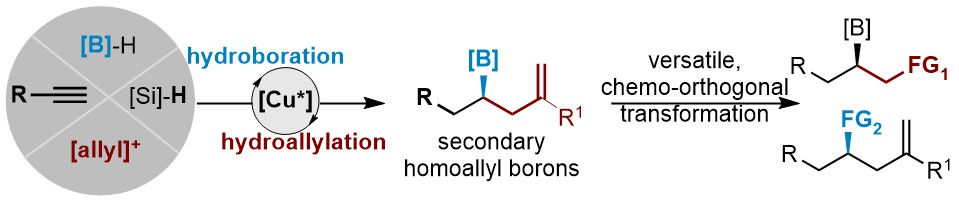
Enantioenriched secondary homoallyl borons─featuring both an alkene and a chiral secondary alkyl borons─are invaluable synthons in organic synthesis.Existing methods often require substrates with pre-installed boryl group or face challenges with regioselectivity. To address these limitations, we developed a simple and straightforward copper-catalyzed enantioselective approach that utilizes simple terminal alkynes as starting materials. For further details and potential applications in total synthesisi of natural products, see our paper.
44. Enantioselective 1,4-Borylative Amination (Invited Manuscript)
Zhu, Yu-Shen; Zhu, Ying-Ying; Guo, Ya-Lin; Su, Bo* Trends in Chemistry, 2024, 7, 101-102.
Selected as "Mechanism of the Month"
43. Enantioselective borylative functionalization of internal alkenes: a platform for constructing vicinal stereocenters (Invited Review)
Zhu, Y.-S.ǂ; Zhao, H.-T.; Li, J.-X.; Su, B.* Chin. J. Chem., 2024, 42, 3588-3604.
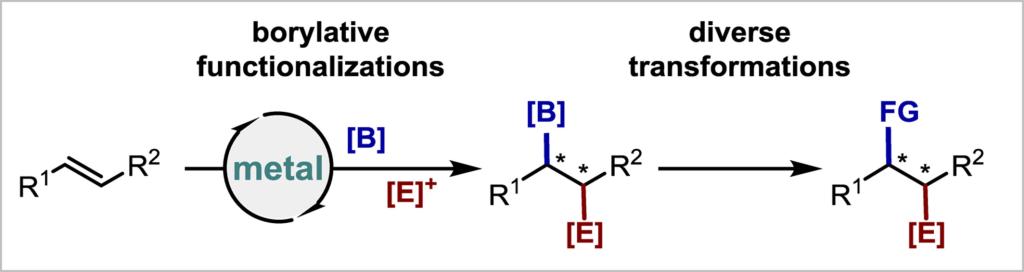
Vicinal stereogenic centers are ubiquitous structural scaffolds in both natural products and synthetic compounds, yet their enantioselective construction remains a significant challenge in organic synthesis. Enantioselective borylative functionalizations of internal alkenes offer a promising strategy for the enantioselective installation of two adjacent structurally diverse chiral centers by leveraging the reactivity of the boryl unit. Check out this review for recent advancements, potential utilities of this method in constructing vicinal stereogenic centers, and unsolved challenges and future directions in this field.
42. Covalent modulation of mPGES1 activity via α,β-unsaturated aldehyde group: Implications for downregulating PGE2 expression and antipyretic response
Fuyun Chi, Man Zhang, Yiman Han, Fukui Shen, Shijie Peng, Su B., Yuanyuan Hou, Gang Bai* Chin. Chem. Lett., 2024, https://doi.org/10.1016/j.cclet.2024.109913
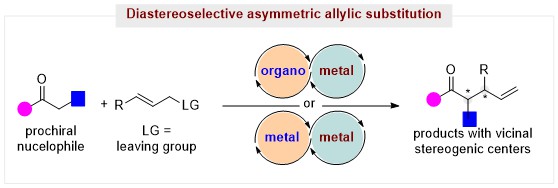
41. Dual-Catalysis-Enabled Construction of Vicinal Stereogenic Centers through Diastereo- and Enantioselective Allylic Substitution (Invited Review). (selected as COVER GRAPHIC)
Yang, K.; Chen, L.; Su B.* Synthesis, 2024, 56, 3365-3376.
40. Iridium-catalyzed, site-selective silylation of secondary Csp3-H bonds in secondary alcohols and ketones
Wilson, J.; Su, B., Yoritate, M., Shi, J., Hartwig, J.* J. Am. Chem. Soc., 2023, 145, 19490-19495
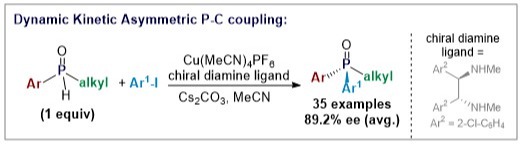
39. Copper-Catalyzed Dynamic Kinetic Asymmetric P‐C Coupling of Secondary Phosphine Oxides and Aryl iodides.
Kang J, Ding K, Ren SM, Su B.*, Angew. Chem. Int. Ed. 2023, 62, e202301628.
Highlighted by Synpacts
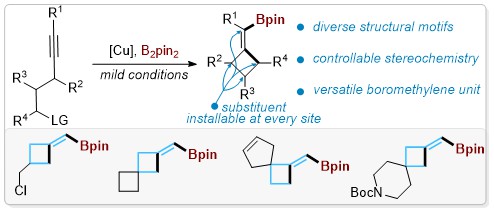
38. A General Catalytic Synthetic Strategy for Highly Strained Methylenecyclobutanes and Spiromethylenecyclobutanes.
Zhao H, Lin Y, Jiang M, Su B.*, Chem. Sci., 2023, 14, 7897-7904.
Highlighted by Synfacts, Chemistry World.
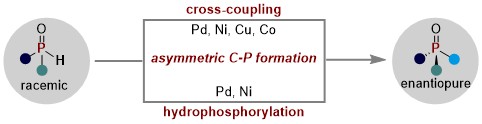
37. Synthesis of P-Stereogenic Compounds by Transition Metal-Catalyzed Asymmetric Transformation of H−P(O) Compounds: Progress, Challenges, and Prospects (Invited Review)
Ding, K.; Su B.*, Eur. J. Org. Chem., 2023, 27, e202301160.
36. Development of Chiral Ligands for the Transition Metal‐catalyzed Enantioselective Silylation and Borylation of C‐H Bonds.
Su B.*, Hartwig J*, Angew. Chem. Int. Ed. 2022, 61, 9, e202113343.
35. Synthesis of P-Chiral Tertiary Phosphine Oxides by Copper-Catalyzed Dynamic Kinetic Asymmetric C–P Cross- Coupling (Invited Synpacts)
Kang J, Ding K, Ren SM, Su B.*, Synlett, 2023, 35, 741-746.
34. Highly Stereoselective Syntheses of α,α-Disubstituted (E)- and (Z)-Crotylboronates.
Zhang, Z.; Liu, J.M.; Gao, S.; Su, B.*; Chen, M.* J. Org. Chem, 2023, 88, 3288.
33. Cu-Catalyzed Highly Stereoselective Syntheses of (E)-δ-Vinyl-homoallylic Alcohols.
Liu, J.M.; Su, B.*; Chen, M.* Org. Lett., 2021, 23, 15, 6035.
32. Diverse functionalization of strong alkyl C–H bonds by undirected borylation.
Oeschger, R.ǂ; Su, B.ǂ; Yu, I.; Ehinger, C.; Romero, E.; He, S.; Hartwig, J. F.*,Science, 2020, 368, 736-741. (ǂEqual contribution)
31. Application of Trimethylgermanyl-Substituted Bisphosphine Ligands with Enhanced Dispersion Interactions to Copper-Catalyzed Hydrobo ration of Disubstituted Alkenes.
Xi, Y.-M.ǂ; Su, B.ǂ; Qi, X.; Pedram, S.; Hartwig, J. F.*, J. Am. Chem. Soc. 2020, 142, 18213-18222. (ǂEqual contribution)

Prior to NKU
30. Palladium-Catalyzed Oxidation of β-C(sp3)–H Bonds of Primary Alkylamines through a Rare Four-Membered Palladacycle Intermediate.
Su, B.; Bunescu, A.; Qiu, Y.-H.; Zuend, S.; Ernst, M.; Hartwig, J. F.*, J. Am. Chem. Soc. 2020,142, 7912-7919.
29. Ir-Catalyzed Intramolecular Silylation of C-H Bonds of Aliphatic Amines.
Su, B.; ǂ Lee T.;ǂ Hartwig, J. F.*, J. Am. Chem. Soc. 2018,140, 18032-18038. (ǂEqual contribution)
28. Ir-Catalyzed, Silyl-Directed, peri-Borylation of C-H Bonds in Fused Polycyclic Arenes and Heteroarenes.
Su, B.; Hartwig, J. F.*, Angew. Chem. Int. Ed. 2018, 57, 10163-10167.
27. Ir-Catalyzed Enantioselective, Intramolecular Silylation of Methyl C–H Bonds.
Su, B.; Hartwig, J. F.*, J. Am. Chem. Soc.2017,139, 12137-12140.
26. Enantioselective Borylation of Aromatic C−H Bonds with Chiral Dinitrogen Ligands.
Su, B.; Zhou, T.-G.; Xu, P.-L.; Shi, Z.-J.; Hartwig, J. F.*, Angew. Chem. Int. Ed. 2017,56, 7205-7208.
25. A Chiral Nitrogen Ligand for Enantioselective, Iridium-Catalyzed Silylation of Aromatic C-H Bonds.
Su, B.; Zhou, T.G.; Li, X.W.; Shao, X.R.; Xu, P.L.; Wu, W.L.; Hartwig, J. F.*; Shi Z.J.*, Angew. Chem. Int. Ed. 2017, 56, 1092-1096.
24. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations.
Su, B.; Cao, Z.-C.; Shi, Z.-J.*, Acc. Chem. Res. 2015, 48, 886-896.
23. Diversity-Oriented Synthesis through Rh-Catalyzed Selective Transformations of a Novel Multirole Directing Group.
Su, B.; Wei, J.B.; Wu, W.L.; Shi, Z.J.*, ChemCatChem 2015, 7, 2986-2990.
22. Silver-catalysed direct amination of unactivated C-H bonds of functionalized molecules.
Yang, M. Y.; Su, B.; Wang, Y.; Chen, K.; Jiang, X. Y.; Zhang, Y. F.; Zhang, X. S.; Chen, G. H.; Cheng, Y.; Cao, Z. C.;
Guo, Q. Y.; Wang, L. S.; Shi, Z. J., Nature Commun. 2014, 5. 4707-4712.
21. Spatial Configuration and Three-Dimensional Conformation Directed Design, Synthesis, Antiviral Activity, and Structure–Activity Relationships of Phenanthroindolizidine Analogues.
Su, B.; Cai, C.; Deng, M.; Wang, Q. M., J. Agric. Food. Chem. 2016, 64, 2039-2045.
20. An enantioselective strategy for the total synthesis of (S)-tylophorine via catalytic asymmetric allylation and a one-pot DMAP-promoted isocyanate formation/Lewis acid catalyzed cyclization sequence.
Su, B.; Zhang, H.; Deng, M.; Wang, Q. M., Org. Biomol. Chem. 2014, 12, 3616-3621.
19. Sodium Nitrite-Catalyzed Aerobic Oxidative Csp2-Csp3Coupling: Direct Construction of the 4-Aryldihydroisoquinolinone Moiety.
Su, B.; ǂDeng, M.;ǂWang, Q. M., Adv. Synth. & Catal. 2014, 356, 977-981. (ǂEqual contribution)
18. Design, synthesis, antiviral activity, and SARs of 13a-substituted phenanthroindolizidine alkaloid derivatives.
Su, B.;ǂ Cai, C. L.;ǂ Deng, M.; Liang, D. M.; Wang, L. Z.; Wang, Q. M., Bioorg. Med. Chem. Lett. 2014, 24, 2881-2884. (ǂEqual contribution)
17. Design, Synthesis, Antiviral Activity, and Structure–Activity Relationships (SARs) of Two Types of Structurally Novel Phenanthroindo/quinolizidine Analogues.
Su, B.;ǂChen, F.;ǂWang, L.; Wang, Q. M., J. Agric. Food. Chem. 2014, 62, 1233-1239. (ǂEqual contribution)
16. Bioinspired Construction of a Spirocyclohexadienone Moiety via Sodium Nitrite Catalyzed Aerobic Intramolecular Oxidative Phenol Coupling.
Su, B.;ǂDeng, M.;ǂWang, Q. M., Org. Lett. 2013,15, 1606–1609. (ǂEqual contribution)
15. A Novel Strategy for the Synthesis of Phenanthroindolizidine alkaloids via one-pot Intramolecular Schmidt/Bischler-Napieralski/ Imine-reduction reaction.
Su, B.;ǂChen, F. Z.;ǂWang, Q. M., J. Org. Chem. 2013,78, 2775–2779. (ǂEqual contribution)
14. The first enantioselective approach to 13a-methyl-14-hydroxyphenanthroindolizidine alkaloids: synthetic studies towards hypoe-stestatin 2.
Su, B.; Deng, M.; Wang, Q. M., Eur. J. Org. Chem. 2013,10, 1979–1985.
13. Enantioselective Approach to 13a-Methylphenanthroindolizidine Alkaloids.
Su, B.; Cai, C. L.; Wang, Q. M., J. Org. Chem. 2012, 77, 7981–7987.
12. A Novel Sodium Nitrite-Catalyzed Oxidative Coupling for Constructing Polymethoxyphenanthrene Rings.
Su, B.; Li, L.; Hu, Y. N.; Liu, Y. X.; Wang, Q. M., Adv. Synth. Catal. 2012, 354, 383–387.
11. Design, synthesis, antivirus activity, and SARs of phenanthroquinolizidine alkaloid derivative
Su, B.; Cai Chunlong; Li, Y., Wang, Q.-M., ACS Agric. Sci. Technol, 1(03); 222-229.
10. Total synthesis of the Proposed Structures of Tyloindane and Its Diastereoisomer
Su, B.; Zhang, H.; Wang, Q.-M., Synthesis, 54(08); 1983-1988.
9. An Unprecedented Cyano-Induced Sodium Nitrite-Catalyzed C (sp3)-H and C (sp2)-H Coupling Reaction Current Organic
L Li, B Su, Y Liu, Q Wang Synthesis 2018, 15, 989-994.
8. Total synthesis of the reported structure of 13a-hydroxytylophorine
H Zhang, G Li, B Su, M Deng, YX Liu, YC Gu, QM Wang Scientific reports 2017, 7, 1-7.
7. Total synthesis of phenanthroindolizidine alkaloids via asymmetric deprotonation of N-Boc-pyrrolidine.
Deng, M.; Su, B.; Zhang, H.; Liu, Y. X.; Wang, Q.-M., Rsc Adv. 2014, 4, 14979-14984.
6. Asymmetric synthesis of (S)-tylophorine and (S)-cryptopleurine via one-pot Curtius rearrangement and Friedel-Crafts reaction tandem sequence.
Chen, F.; Su, B.; Wang, Q.-M., Org. Chem. Front. 2014, 1, 674-677.
5. First total synthesis of (+)-6-O-desmethylantofine.
Wu, M.; Li, L.; Su, B.; Liu, Z. H.; Wang, Q., Org. Biomol. Chem. 2011, 9, 141-145.
4. “First total synthesis of Papilistatin.”
Wu, M.; Li, L.; Feng, A. Z.; Su, B.; Liang, D. M.; Liu, Y. X.; Wang, Q.-M.,Org. Biomol. Chem. 2011, 9, 2539-2542.
3. Synthesis and antiviral activities of phenanthroindolizidine alkaloids and their derivatives.
Wang, K. L.; Su, B.; Wang, Z. W.; Wu, M.; Li, Z.; Hu, Y. N.; Fan, Z. J.; Mi, N.; Wang, Q.-M., J. Agric Food Chem. 2010, 58, 2703-2709.
2. “m-CPBA/TFA an efficient nonmetallic reagent for oxidative coupling of 1,2-diarylethylenes.”
Wang, K. L.; Hu, Y. N.; Wu, M.; Li, Z.; Liu, Z. H.; Su, B.; Yu, A.; Liu, Y.; Wang, Q.-M., Tetrahedron. 2010, 66, 9135-9140.
1. A simple and efficient oxidative coupling of aromatic nuclei mediated by manganese dioxide.
Wang, K. L.; Hu, Y. N.; Li, Z.; Wu, M.; Liu, Z. H.; Su, B.; Yu, A.; Liu, Y.; Wang, Q.-M., Synthesis. 2010, 7, 1083-1090.